Family: Nimaviridae
Han-Ching Wang, Ikuo Hirono, Mary Beth Bacano Maningas, Kunlaya Somboonwiwat and Grant Stentiford
The citation for this ICTV Report chapter is the summary published as Wang et al., (2019):
ICTV Virus Taxonomy Profile: Nimaviridae, Journal of General Virology, 100: 1053–1054.
Corresponding author: Han-Ching Wang ([email protected])
Edited by: Balázs Harrach and Andrew J. Davison
Posted: February 2019
PDF: ICTV_Nimaviridae.pdf
Summary
The family Nimaviridae includes the single genus Whispovirus and the single species, White spot syndrome virus isolates of which infect a wide range of aquatic crustaceans and cause substantial economic losses (Table 1.Nimaviridae). Virions are ellipsoid to bacilliform with a terminal extension, and contain more than 40 structural proteins ranging in mass from 11 to 664 kDa. These assemble into three structural layers (an outer envelope, a tegument and a nucleocapsid) that surround the core genomic DNA. The circular dsDNA genome is 280–307 kbp and contains several homologous repeat regions. Despite some genotypic variability, all isolates are classified within the same species.
Table 1. Nimaviridae. Characteristics of members of the family Nimaviridae.
| Characteristic | Description |
| Typical member | white spot syndrome virus-CN (AF332093), species White spot syndrome virus, genus Whispovirus |
| Virion | Ellipsoid-to-bacilliform virion consisting of envelope, tegument, rod-shaped nucleocapsid and, sometimes, a thread-like terminal extension, 70–170 nm × 210–420 nm, containing >40 structural proteins |
| Genome | A single circular dsDNA of 280–307 kbp with 9 internal homologous repeat regions |
| Replication | Formation of the nucleocapsid and assembly of the intact virion occur in the nucleus; only virus protein translation occurs in the cytoplasm |
| Translation | Most virus mRNAs are capped, polyadenylated and translated via the canonical cap-dependent pathway; some genes, including structural genes, are expressed via mRNAs translated by the canonical cap-independent pathway using internal ribosome entry site elements |
| Host range | Wide range of crustaceans (order Decapoda) from marine, brackish or freshwater sources |
| Taxonomy | Class Naldaviricetes: single genus and species |
Virion
Morphology
Virions of white spot syndrome virus (WSSV) are enveloped, ellipsoid-to-bacilliform particles of 70–170 nm in width and 210–420 nm in length (Table 1.Nimaviridae and Figure 1. .Nimaviridae). A thread or flagellum-like projection (or extension) is present at one end of most virions (Chou et al., 1995, Durand et al., 1997, Lu et al., 1997). The virion consists of an inner, rod-shaped nucleocapsid with a tight-fitting capsid layer, an intermediate tegument layer and an outer lipid-containing trilaminar envelope (Tsai et al., 2006). In virions purified from the crayfish Procambarus clarkii, the virion lipids are apparently derived from host cell nuclei (Zhou et al., 2008). There is currently no evidence to suggest that the lipid components of virions are derived from the host cell membrane. The purified nucleocapsid is 50–80 nm in width and 300–420 nm in length.
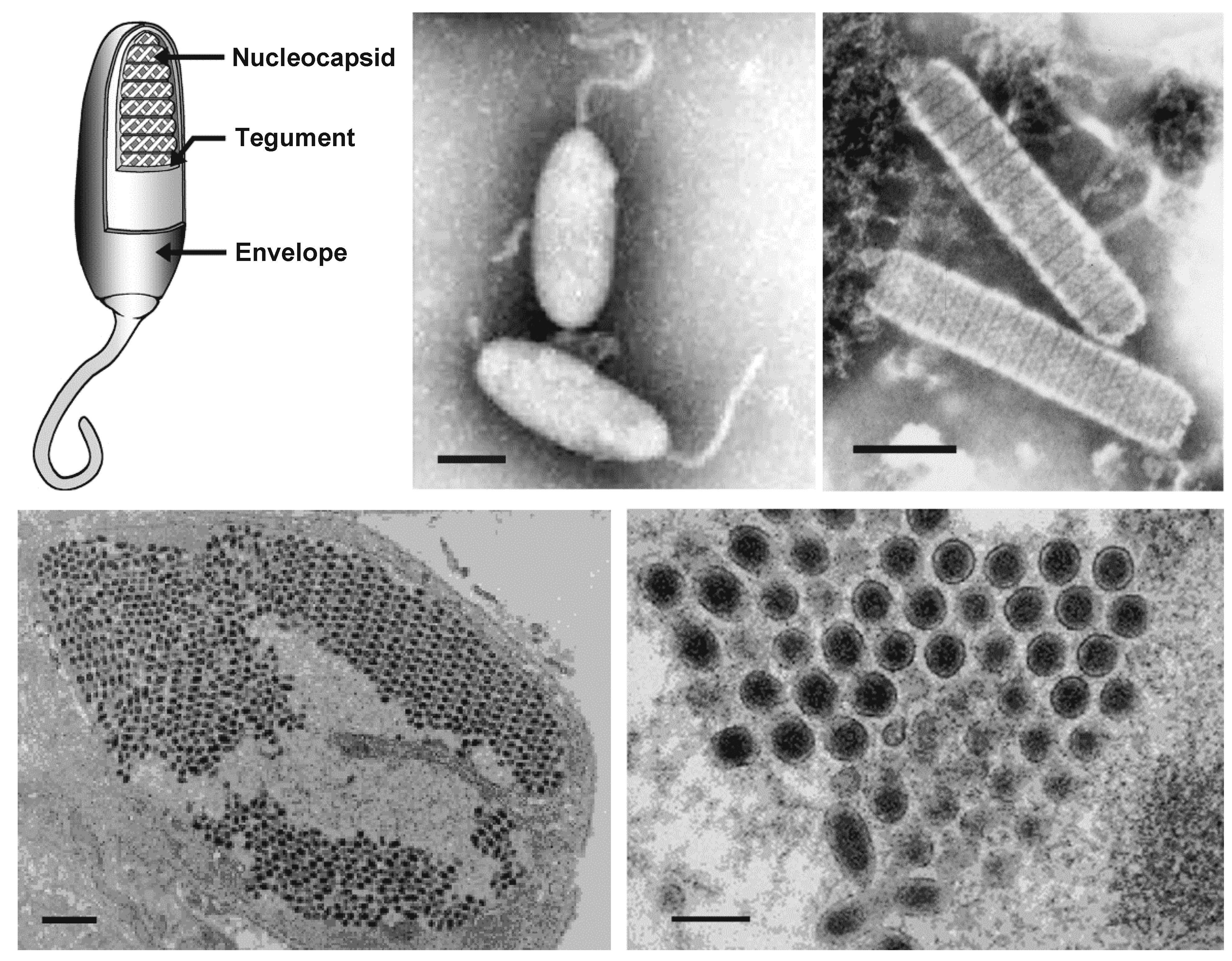 |
| Figure 1. .Nimaviridae. (Top) Morphology of virions of white spot syndrome virus (WSSV). (Left) Schematic illustration of the structure of a typical whispovirus virion using a cutaway section to show the locations of major virion proteins. (Top center and right) Negative-contrast electron micrographs of WSSV virions (center, courtesy of Marielle van Hulten) and nucleocapsids (right, courtesy of Don Lightner) from hemolymph of infected Penaeus monodon. The bars represent 100 nm. (Bottom left) Thin section of WSSV-infected stomach epithelium showing the parallel arrangement of virions in the nucleoplasm (the bar represents 1 µm) and (bottom right) a cross-section of virions (the bar represents 100 nm) (courtesy of Don Lightner). |
Physicochemical and physical properties
WSSV virions have a buoyant density of 1.22 g cm−3 in CsCl, whereas nucleocapsids have a buoyant density of 1.31 g cm−3. Virions are viable for at least 1 month at 30°C in seawater under laboratory conditions and in ponds for at least 3–4 days. They are inactivated by pH <3.0 for 1 h and pH 12 for 10 min, exposure to UV irradiation at 9.3 x 105 µW s/cm2 for 1 h, and by incubation for <1 min at 60°C. Virions are also sensitive to sodium hypochlorite, benzalkonium chloride, iodophore and ozone (Chang et al., 1998, Nakano et al., 1998, Balasubramanian et al., 2006).
Nucleic acid
The nucleocapsid contains a single molecule of circular dsDNA of 280–307 kbp. The G+C content of WSSV genome DNA is about 41% (van Hulten et al., 2001, Yang et al., 2001).
Proteins
High-throughput proteomic analysis indicates that WSSV virions contain more than 40 structural proteins ranging in mass from 11 to 664 kDa and arranged in three structural layers that surround the core genomic DNA: an outer envelope, a tegument and a nucleocapsid (Table 2. Nimaviridae) (Tsai et al., 2006, Tsai et al., 2004, Xie et al., 2006). There are six major structural proteins: VP28 and VP19 in the envelope; VP26 and VP24 in the tegument; and VP664 and VP15 in the nucleocapsid. The three major proteins, VP28, VP26 and VP24, are phylogenetically related. VP664, which has a theoretical mass of 664 kDa, is the largest viral structural protein known. It forms unique stacked rings in the nucleocapsid (Leu et al., 2005). VP15 is a very basic, histone-like DNA-binding nucleoprotein (Witteveldt et al., 2005). Two structural proteins, VP38B and VP36A, also block shrimp caspase-induced apoptosis (Leu et al., 2010, Bowornsakulwong et al., 2017). One envelope protein, VP22, shows relatively high similarity to TATA-box binding protein. WSSV458 is a tegument protein that can bind to the shrimp protein PmVRP15 to facilitate viral trafficking and assembly (Jaree et al., 2016). Other WSSV structural proteins, including three envelope proteins (WSSV189, VP39 and WSSV471), a nucleocapsid protein (WSSV186) and a tegument protein (WSSV458), are targeted by the shrimp antimicrobial peptide, ALFPm3 (Suraprasit et al., 2014, Methatham et al., 2017). Curiously, silencing of the genes encoding VP28, VP26, VP19 and VP15 by RNA interference also leads to a reduction in WSSV replication (Escobedo-Bonilla 2011).
Table 2. Nimaviridae. WSSV-encoded structural proteins.
| No. | Sub-category | Protein namea | WSSV ORF | |
WSSV-CN (AF332093) | WSSV-TW (AF440570) | |||
| 1 | Envelope proteinsb
| VP187 | wsv209 | wssv264 |
| 2 | VP180 | wsv001 | wssv052 | |
| 3 | VP150 | wsv011 | wssv067 | |
| 4 | VP136B | wvs465 | wssv524 | |
| 5 | VP124 | wsv216 | wssv271 | |
| 6 | VP110 | wsv035 | wssv092 | |
| 7 | VP90 | wsv327 | wssv383 | |
| 8 | VP75 | wsv332 | wssv388 | |
| 9 | VP56 (VP60A) | wsv325 | wssv381 | |
| 10 | VP55 | wsv526 | wssv051 | |
| 11 | VP53A | wsv011 | wssv067 | |
| 12 | VP53B | wsv115 | wssv171 | |
| 13 | VP53C | wsv269 | wssv324 | |
| 14 | VP52A (VP51A) | wsv238 | wssv294 | |
| 15 | VP52B (VP51B) | wsv256 | wssv311 | |
| 16 | VP41A | wsv237 | wssv293 | |
| 17 | VP41B | wsv242 | wssv298 | |
| 18 | VP39 (VP39B) | wsv339 | wssv395 | |
| 19 | VP38 (VP38A) | wsv259 | wssv314 | |
| 20 | VP38B | wsv390 | wssv449 | |
| 21 | VP33 (VP36B) | wsv254 | wssv309 | |
| 22 | VP32 | wsv198 | wssv253 | |
| 23 | VP31 | wsv340 | wssv396 | |
| 24 | VP28 | wsv421 | wssv480 | |
| 25 | VP22 | wsv303 | wssv359 | |
| 26 | VP19 | wsv414 | wssv473 | |
| 27 | VP16 (VP13B) | wsv321 | wssv377 | |
| 28 | VP14 | wsv293a | wssv349 | |
| 29 | VP13A | wsv284 | wssv339 | |
| 30 | VP12 (VP12A) | wsv009 | wssv065 | |
| 31 | VP12B | wsv386 | wssv445 | |
| 32 | VP11 | wsv338 | wssv394 | |
| 33 | WSSV189 | wsv134 | wssv189 | |
| 33 | WSSV471 | wsv412 | wssv471 | |
| 34 | Tegument proteins | VP95 | wsv442 | wssv502 |
| 35 | VP39A | wsv306 | wssv362 | |
| 36 | VP36A | wsv077 | wssv134 | |
| 37 | VP26 | wsv311 | wssv367 | |
| 38 | VP24 | wsv002 | wssv058 | |
| 39 | WSSV458 | wsv399 | wssv458 | |
| 40 | Nucleocapsid proteins | VP664 | wsv360 | wssv419 |
| 41 | VP190 (VP160A) | wsv289 | wssv344 | |
| 42 | VP160B | wsv037 | wssv094 | |
| 43 | VP136 (VP136A) | wsv271 | wssv326 | |
| 44 | VP76 (VP73) | wsv220 | wssv275 | |
| 45 | VP60 (VP60B) | wsv415 | wssv474 | |
| 46 | VP51 (VP51C) | wsv308 | wssv364 | |
| 47 | VP15 | wsv214 | wssv269 | |
| 48 | WSSV186 | wsv131 | wssv186 | |
a: Structural protein names are from (Tsai et al., 2004, Tsai et al., 2006, Xie et al., 2006). Some structural proteins have alternative names; these aliases are shown in parentheses (Tsai et al., 2004, Tsai et al., 2006).
b: Some proteins listed as envelope proteins may in fact turn out to be tegument proteins.
Lipids
Virion lipid components include neutral lipids and the phospoholipids phosphatidylcholine and phosphatidylethanolamine (Zhou et al., 2008). A GC/MS analysis of the fatty acids in crayfish stomach and WSSV virions suggested that, although the respective compositions of their long chain fatty acids are broadly similar, WSSV virions contain more saturated C18:0 (stearic acid) and less C20:5n-3 (eicosapentaenoic acid) and C22:6n-3 (docosahexaenoic acid) fatty acids (Hsieh et al., 2015).
Carbohydrates
None of the major virion proteins is glycosylated.
Genome organization and replication
Genotypic variants exist that can be distinguished by restriction length polymorphism and genomic sequence data. Several WSSV isolates have been sequenced, and their genomes are variously 280,591 bp (WSSV-IN_AP4RU; MG702567), 297,967 bp (WSSV-TH; AF369029), 305,119 bp (WSSV-CN; AF332093) and 307,287 bp (WSSV-TW; AF440570). In the case of WSSV-TH, major differences can be attributed to deletions between positions 275,238 and 287,285, and between 267,203 and 268,046, and also to insertion of a 1.3 kb transposase sequence in the WSSV-TW genome at positions 204,978–204,979. The WSSV genome is further characterized by the presence of nine homologous repeat (Hr) regions (Hr1-9; Figure 2. .Nimaviridae). The number of imperfect palindromic repeats (each of 250 bp) within an Hr varies among isolates (Marks et al., 2004).
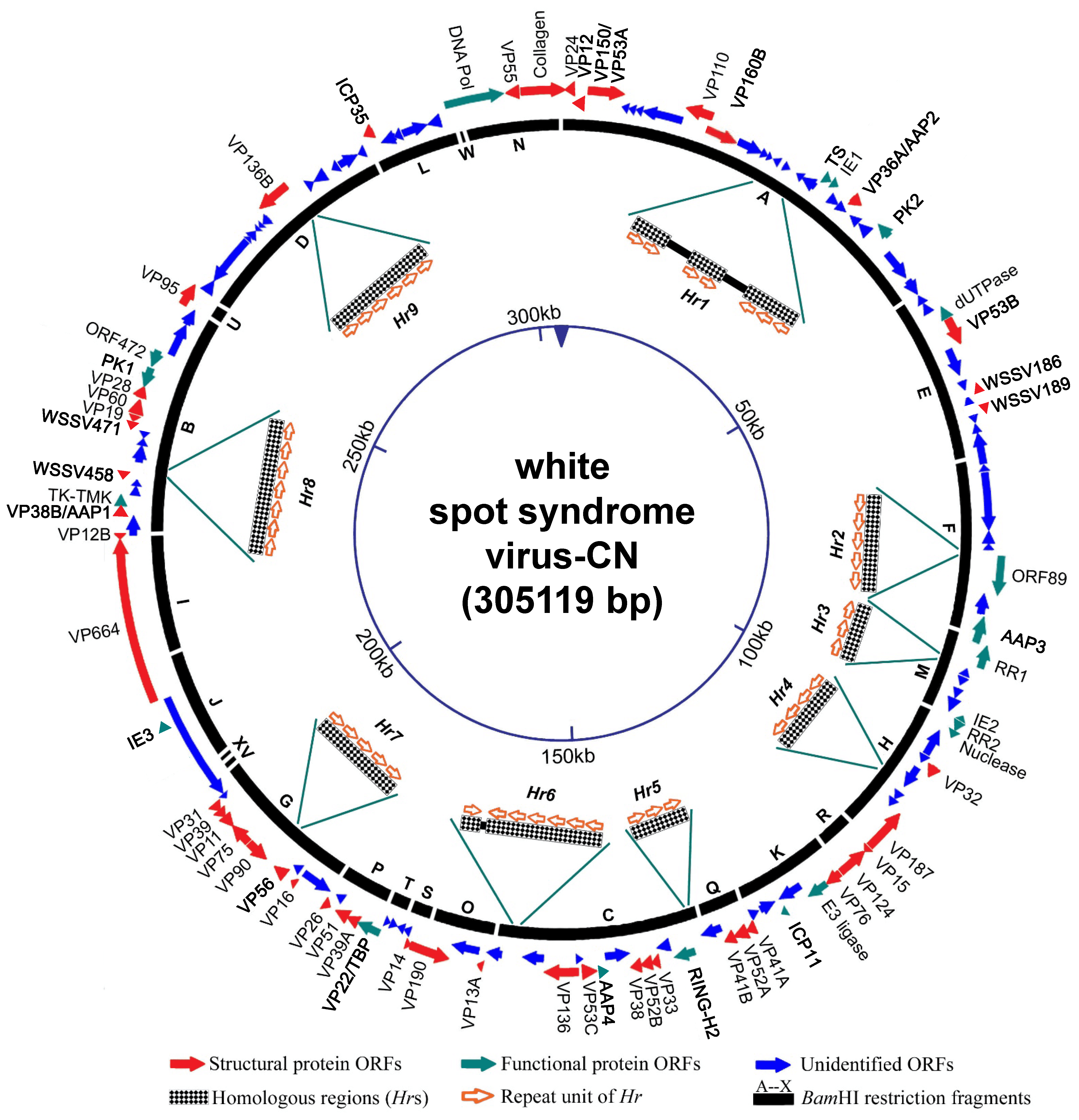 |
| Figure 2. .Nimaviridae. Schematic map of the circular dsDNA genome of white spot syndrome virus type strain CN. Solid arrows indicate the positions and direction of transcription of ORFs. |
WSSV enters host cells via caveola- or clathrin-mediated endocytosis (Huang et al., 2013, Huang et al., 2015). After entering, WSSV triggers host metabolic changes in order to complete its replication, affecting aerobic glycolysis, glutaminolysis and lipid metabolism (Hsieh et al., 2015, Su et al., 2014, Li et al., 2016, Chen et al., 2016, Fan et al., 2016). Transcription of virus mRNA, replication of virus DNA, formation of nucleocapsids and assembly of virions take place in the nucleus; virus proteins are translated in the cytoplasm (Leu et al., 2009). Transcription of WSSV genes is temporally regulated, and four main gene classes are assigned according to the time of mRNA expression: immediate early (IE), early, late and very late genes. Most virus mRNA is 5′-capped and 3′-polyadenylated. There is no evidence for RNA splicing. The virion protein genes are dispersed on both strands of the genome, and some, such as vp31/vp39b/vp11, are arranged in clusters with non-structural genes. These produce polycistronic mRNAs and use an IRES (internal ribosome entry site element) mechanism to regulate translation. Several IRES elements have been identified in the WSSV genome: upstream from vp28, upstream from wsv493 (icp35) and an extension across the coding regions of vp31 and vp39b (Han and Zhang 2006, Kang et al., 2009, Kang et al., 2013).
WSSV ie1 has a promoter motif ([A/C][A/C]TCANT) that matches the RNA polII core promoter in arthropods. The WSSV late gene consensus promoter (A[A/T][A/T/G]AC) is usually situated less than 100 bp upstream from the transcriptional start site. Consensus TATA box sequences have been found about 25 bp upstream from early gene transcription initiation sites. Late transcripts seem to start 25 bp downstream from an A- or T-rich region (Marks et al., 2006).
Genomic analysis reveals that, of the 531 ORFs of 60 codons or more in the WSSV genome, only 181 seem likely to encode functional proteins (WSSV-CN; Figure 2. .Nimaviridae). Only a few functional genes have been identified: these encode virion proteins, proteins involved in initiating virus replication (IE proteins), proteins involved in virus DNA replication (DNA polymerase, ribonucleotide reductase subunits [RR1 and RR2], dUTPase, thymidylate synthase [TS], thymidine kinase-thymidylate kinase [TK-TMK]), protein-modifying proteins (protein kinase), anti-apoptotic proteins and a histone-binding DNA mimic, ICP11 (Tables 2. Nimaviridae and 3. Nimaviridae). A total of 18 IE genes have been proposed. IE1 has a transactivation activity and interacts with shrimp TATA box-binding protein, STAT and Yin Yang 1 protein (Liu et al., 2011, Yao et al., 2016, Huang et al., 2017b). WSSV164 and WSSV453 are anti-melanization proteins that interact with proteins involved in the proPO system (Sutthangkul et al., 2017, Sangsuriya et al., 2018). Four genes, AAP1–AAP4, show anti-apoptotic activity (Leu et al., 2010, Bowornsakulwong et al., 2017, He et al., 2009, Lertwimol et al., 2014). WSV152 is a mitochondrion-targeting protein that localizes specifically on the outer mitochondrial membrane (Yan et al., 2016). The most highly expressed WSSV nonstructural protein is ICP11 (Wang et al., 2008).
Table 3. Nimaviridae. WSSV-encoded non-structural proteins
| No. | Sub-category | WSSV ORF | |||
| Protein name | WSSV-CN (AF332093) | WSSV-TW (AF440570) | |||
| 1 | IE proteins | IE1 | wsv069 | wssv126 | |
| 2 | IE2 | wsv187. | wssv242 | ||
| 3 | IE3 | wsv359 | wssv418 | ||
| 4 | Ubiquitin E3 ligase | wsv249 | wssv304 | ||
| 5 | IE candidate | wsv051 | wssv108 | ||
| 6 | IE candidate | wsv078 | wssv135 | ||
| 7 | IE candidate | wsv079 | wssv136 | ||
| 8 | IE candidate | wsv080 | wssv137 | ||
| 9 | IE candidate | wsv083 | wssv140 | ||
| 10 | IE candidate | wsv091 | wssv148/149 | ||
| 11 | IE candidate | wsv094 | wssv150 | ||
| 12 | IE candidate | wsv098 | wssv154 | ||
| 13 | IE candidate | wsv099 | wssv155 | ||
| 14 | IE candidate | wsv100 | wssv156 | ||
| 15 | IE candidate | wsv101 | wssv157 | ||
| 16 | IE candidate | wsv103 | wssv159 | ||
| 17 | IE candidate | wsv108 | wssv164 | ||
| 18 | IE candidate | wsv178 | wssv234 | ||
| 19 | Anti-apoptotic proteins (AAP) | AAP1 | wsv390 | wssv449 | |
| 20 | AAP2 | wsv077 | wssv134 | ||
| 21 | AAP3 | wsv166 | wssv222 | ||
| 22 | AAP4 | wsv267 | wssv322 | ||
| 23 | Anti-melanization proteins | WSSV164 | wsv108 | wssv164 | |
| 24 | WSSV453 | wsv394 | wssv453 | ||
| 25 | Latency-related genes | ORF89 | wsv151 | wssv207 | |
| 26 | ORF427 | wsv427 | wssv486 | ||
| 27 | Enzymes
| Thymidylate synthase (TS) | wsv067 | wssv124 | |
| 28 | Protein kinase 2 (PK2) | wsv083 | wssv140 | ||
| 29 | dUTPase | wsv112 | wssv168 | ||
| 30 | Ribonucleotide reductase 1 (RR1) | wsv172 | wssv228 | ||
| 31 | Ribonucleotide reductase 2 (RR2) | wsv188 | wssv243 | ||
| 32 | Nuclease | wsv191 | wssv246 | ||
| 33 | Thymidine kinase thymidylate kinase (TK-TMK) | wsv395 | wssv454 | ||
| 34 | Protein kinase 1 (PK1) | wsv423 | wssv482 | ||
| 35 | DNA polymerase | wsv514 | wssv037 | ||
| 36 | Others | RING-H2 protein | wsv194. | wssv249 | |
| 37 | Collagen-like protein | wsv001 | wssv052 | ||
| 38 | Histone-binding DNA mimic (ICP11) | wsv230 | wssv285 | ||
| 39 | Mitochondria targeting protein (MTP) | wsv152 | wssv208 | ||
| 40 | TATA-box binding protein (TBP) | wsv303 | wssv359 | ||
| 41 | ICP35 | wsv493 | wssv019 | ||
To regulate its replication, WSSV expresses several virus microRNAs that target either its own genes or those of the host. WSSV-miR-N32 targets wsv459 and wsv322 to suppress virus replication, possibly as a strategy to avoid stimulating host innate immunity (He et al., 2017). Conversely, WSSV-miR-66 and WSSV-miR-68 promote virus replication by targeting wsv094 and wsv 177, and wsv248 and wsv309, respectively (He et al., 2014). WSSV-miR-N13 and WSSV-miR-N23 target Dorsal, the key gene of the Toll pathway, and this results in suppression of Toll-mediated shrimp immunity (Ren et al., 2017), while WSSV-miR-22 targets the shrimp STAT gene to promote WSSV replication (Ren et al., 2015). Shrimp microRNAs also regulate WSSV replication by targeting WSSV genes. The expression of shrimp miR-965 decreases WSSV copy number and WSSV-induced mortality by targeting the wsv240 gene (Shu et al., 2016). Shrimp miR-10A enhances the translation of at least three WSSV genes (vp26, vp28 and wssv102) by targeting the 5′-UTRs of these genes (Huang et al., 2017a).
WSSV has also been shown to contain genes homologous to those of its hosts Marsupenaeus japonicus (Dang et al., 2010) and Penaeus monodon. These may be instances of mimicry of the structure and function of host genes, e.g. to avoid detection and destruction by the host immune system, a stratagem used by other dsDNA viruses. Alternatively, the host genes may be degenerate proviral remnants.
Biology
Host range
WSSV can infect a wide range of aquatic crustaceans, including salt, brackish and fresh water penaeids, crabs, crayfish and hermit crabs (Lo et al., 1996, Flegel 2006, Sánchez-Paz 2010).
Transmission
The virus is transmitted by cannibalism of diseased individuals or via water passing through the gills. The virus can also be transmitted vertically from adults to offspring by being released from non-viable WSSV-infected eggs or from supporting cells in ovarian tissue (Lo et al., 1997).
Geographical distribution
WSSV has been identified from crustaceans in China, Japan, Korea, Southeast Asia, South Asia, the Middle East and the Americas. An unusual property of the virus is the extremely wide host range despite a very low level of genetic polymorphism. The latter suggests that white spot disease (WSD) is a relatively recent epizootic (Nadala and Loh 1998, Pradeep et al., 2012).
Cytopathic effects
The major targets of WSSV infection are tissues of ectodermal and mesodermal embryonic origin, such as subcuticular epithelial cells (including those of the shrimp stomach), gills, lymphoid organ and connective tissue. Although WSSV infects the underlying connective tissue in the shrimp hepatopancreas and midgut, the columnar epithelial cells of these two organs are of endodermal origin and do not become infected. WSSV can be propagated in primary cultures of lymphoid organ and ovary. Whether or not infection causes disease depends upon factors that are poorly understood but related to species tolerance and environmental triggers. Shrimp and other crustaceans susceptible to disease from this virus show gross signs of lethargy, such as lack of appetite, slow movement and often a reddish coloration of the whole body together with “white spots” embedded within the exoskeleton. These spots are the result of calcified deposits that range in diameter from 0.5 to 3.0 mm. However, in some cases, such as acute experimental infections resulting from injected virus preparations, there may be no gross signs of infection other than lethargy and lack of appetite. Most affected animals die 3–10 days after infection. With a lower infection dose to allow sufficient time before mortality, animals susceptible to disease have large numbers of virions circulating in the hemolymph, but this may also occur for tolerant species showing no mortality. Thus, high virus loads per se do not cause disease or mortality in all susceptible species (Durand et al., 1997, Leu et al., 2009, Lo et al., 1996, Sánchez-Paz 2010, Lo et al., 1997).
Antigenicity
Polyclonal and monoclonal antibodies have been raised against WSSV virions, and these can be used as diagnostic tools. Viruses can be neutralized with antisera against the WSSV structural proteins VP12B, VP19, VP28, VP31, VP36A, VP33 (VP36B) and VP51 (Wu et al., 2005, Li et al., 2006, Patil et al., 2011). Recombinant proteins have been widely used as vaccines against WSSV infection. In shrimps, vaccines containing recombinant WSSV structural proteins VP19, VP26, VP28 and VP41A have been shown to improve survival after WSSV infection (Chang et al., 2018). It can be inferred from this that these proteins are involved in WSSV infection.
Derivation of names
Nima: Greek for “thread”; referring to the thread- or tail-like polar extension (appendage) on some virus particles.
The name white spot syndrome virus has been arrived at after the use of various earlier names, such as Chinese baculo-like virus, hypodermic and hematopoietic necrosis baculovirus, Penaeus monodon non-occluded baculovirus II, Penaeus monodon non-occluded baculovirus III, Penaeus monodon rod-shaped nuclear virus, systemic ectodermal and mesodermal baculovirus, white spot bacilliform virus and white spot baculovirus.
Relationships within the family
Despite some genotypic variability, the amino acid sequences of DNA polymerase from geographically distinct WSSV isolates are approximately 99% identical, and all isolates are classified in the same species. Various isolates with small genetic polymorphisms have been identified (variants). Because of the high level of similarity among variants, their evolutionary histories have not yet been clearly established.
Relationships with other taxa
Morphologically, virions and rod-shaped nucleocapsids resemble the budded virus particles of baculoviruses, polydnaviruses and the alphanudivirus Oryctes rhinoceros nudivirus. The presence of Hrs dispersed throughout the genome is a property shared with members of the families Baculoviridae, Ascoviridae, Hytrosaviridae and Nudiviridae. Phylogenetic analysis based on the DNA polymerase from WSSV and a number of other dsDNA viruses shows that WSSV is phylogenetically highly distinct.

