Family: Picobirnaviridae
Bernard Delmas, Houssam Attoui, Souvik Ghosh, Yashpal S. Malik, Egbert Mundt and Vikram N. Vakharia
The citation for this ICTV Report chapter is the summary published as Delmas et al., (2019):
ICTV Virus Taxonomy Profile: Picobirnaviridae, Journal of General Virology, 100, 133–134.
Corresponding author: Bernard Delmas ([email protected])
Edited by: Jens H. Kuhn and Stuart G. Siddell
Posted: October 2018
PDF: ICTV_Picobirnaviridae.pdf
Summary
Picobirnaviridae is a family of viruses with bisegmented (rarely unsegmented) dsRNA genomes comprising about 4.1–4.6 kbp in total, with small non-enveloped spherical virions (Table 1. Picobirnaviridae). The family includes one genus (Orthopicobirnavirus) grouping three genetic clusters with high sequence variability, two defined by viruses infecting vertebrates, and a third with viruses found in invertebrates. Recent bioinformatic analyses have indicated that the members of the family Picobirnaviridae likely consist of bacteriophages, and potentially fungal viruses. The Study Group is investigating a reorganisation of the taxonomy in light of this evidence.
Table 1. Picobirnaviridae. Characteristics of the family Picobirnaviridae.
| Characteristic | Description |
| Typical member | human picobirnavirus, Hy005102 (RNA1: AB186897;RNA2: AB186898), species Orthopicobirnavirus hominis |
| Virion | Non-enveloped, spherical virion, 33–37 nm in diameter |
| Genome | Two double-stranded RNA segments, of 1.7–1.9 kbp and 2.4–2.7 kbp |
| Replication | Not known due to lack of infection models in cell culture or animals |
| Translation | Not known |
| Host range | Vertebrates and invertebrates - see note in Summary above |
| Taxonomy | Realm Riboviria, kingdom Orthornavirae, phylum Pisuviricota, class Duplopiviricetes, order Durnavirales: one genus with three species |
Virion
Morphology
Virus particles are non-enveloped, spherical, 33–37 nm in diameter, with a capsid layer surrounding the genomic dsRNA segments (Pereira et al., 1988). Owing to the absence of a cell culture system for propagating picobirnaviruses, knowledge of virion protein composition comes from virion-like particles produced by recombinant expression of the gene encoding the capsid protein precursor. The virus-like particles have a triacontahedral (30-sided) organization made of 60 symmetric capsid protein (CP) dimers (Duquerroy et al., 2009). The structure reveals an intricate interface with the N-terminal residues exchanged between the two subunits of the dimer. Two domains can be recognized in CP, namely a shell domain and a projection domain that forms the protrusions that stand out in the three-dimensional reconstruction (Figure 1. Picobirnaviridae).
 |
| Figure 1. Picobirnaviridae. Structure of the picobirnavirus particle. (Left) Surface rendering from a three-dimensional reconstruction viewed down a slightly mis-oriented five-fold axis. Note the presence of 60 dimeric protrusions (courtesy of L. Lepault). (Middle) Diagram representing a triacontahedron, a convex polyhedron made of 30 rhombic faces or diamond tiles. (Right) Triacontahedral design of the particle, with each of the tiles formed by two capsid protein dimers colored differently (courtesy of S. Duquerroy). |
Physicochemical and physical properties
Virion buoyant density in CsCl is 1.38 to 1.40 g cm-3.
Nucleic acid
Virions contain two unrelated, linear dsRNA segments (dsRNA1 and dsRNA2) (Pereira et al., 1988). The larger segment (dsRNA1) is 2.4–2.7 kbp with two or three ORFs (Green et al., 1999, Rosen et al., 2000, Wakuda et al., 2005). The smaller segment (dsRNA2) is 1.7–1.9 kbp and is monocistronic. It encodes the viral RNA-dependent RNA polymerase (RdRP). Several picobirnavirus genomes belonging to different genetic clusters are unsegmented with dsRNA2 fused to the 3′-end of dsRNA1 (Li et al., 2015, Shi et al., 2016).
Proteins
Proteolytic maturation of the capsid protein precursor generates an N-terminal peptide rich in basic residues as well as the mature CP (Duquerroy et al., 2009). An additional protein containing repeats of the ExxRxNxxxE motif is translated from dsRNA1 (Duquerroy et al., 2009, Da Costa et al., 2011). The RdRP is translated from dsRNA2 (Collier et al., 2016).
Genome organization and replication
About twenty complete genomic sequences of vertebrate picobirnaviruses and seven from invertebrate picobirnaviruses are available in sequence databases (see Table 2). Most of these isolates have bipartite genomes. The smaller segment (dsRNA2; 1.7–1.9 kbp) encodes an RdRP, whereas the larger (dsRNA1, 2.4–2.7 kbp) possesses two large open reading frames (ORF) that can be preceded by a small ORF (Wakuda et al., 2005, Woo et al., 2012, Bodewes et al., 2013). The dsRNA1 of human picobirnavirus Hy005102, an isolate of the species Human picobirnavirus, has two large ORFs of 224 (ORF2) and 552 (ORF3) codons, preceded by a shorter one (ORF1) of 39 codons (Figure 2. Picobirnaviridae). The three ORFs overlap by eight (ORF1-ORF2 junction) and one (ORF2-ORF3 junction) nucleotides (nt) (Wakuda et al., 2005). The functionality of ORF1 is unclear and ORF2 encodes a protein of unknown function, but ORF3 encodes a precursor of CP which is auto-catalytically cleaved at about 50–70 residues from its N-terminus to generate a positively-charged peptide and mature CP (illustrated for rabbit picornavirus in Figure 3. Picobirnaviridae); (Duquerroy et al., 2009). dsRNA2 encodes a RdRP with a core component containing the canonical A–B–C motif arrangement of the palm subdomain present in conventional nucleic acid polymerases (Collier et al., 2016).
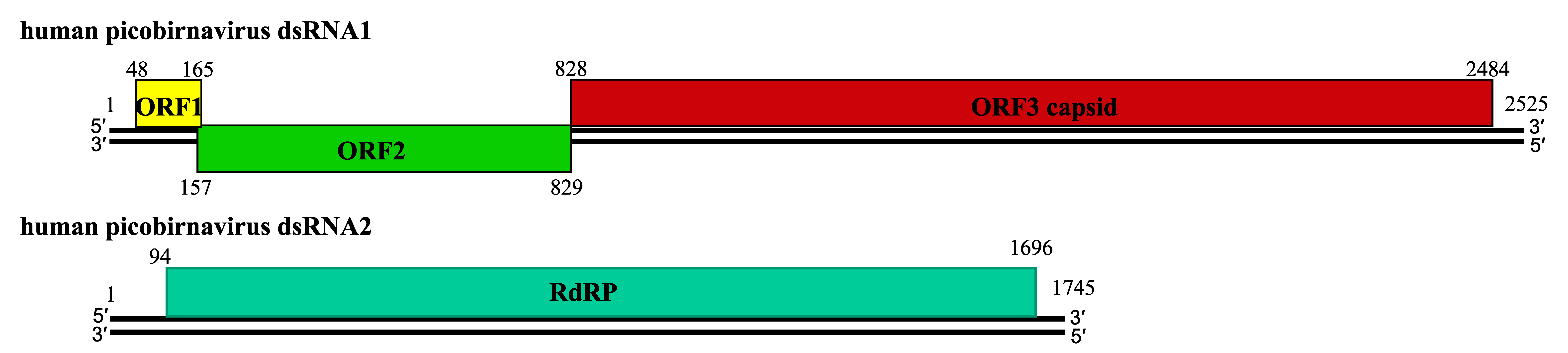 |
| Figure 2. Picobirnaviridae. Schematic representation of the gene arrangement in dsRNA1 and dsRNA2 of human picobirnavirus strain Hy005102 (RNA1:AB186897; RNA2:AB186898). dsRNA1 possesses three ORFs. ORF3 encodes the capsid protein precursor. dsRNA2 encodes an RNA-dependent RNA polymerase. Numbers indicate nucleotide positions. |
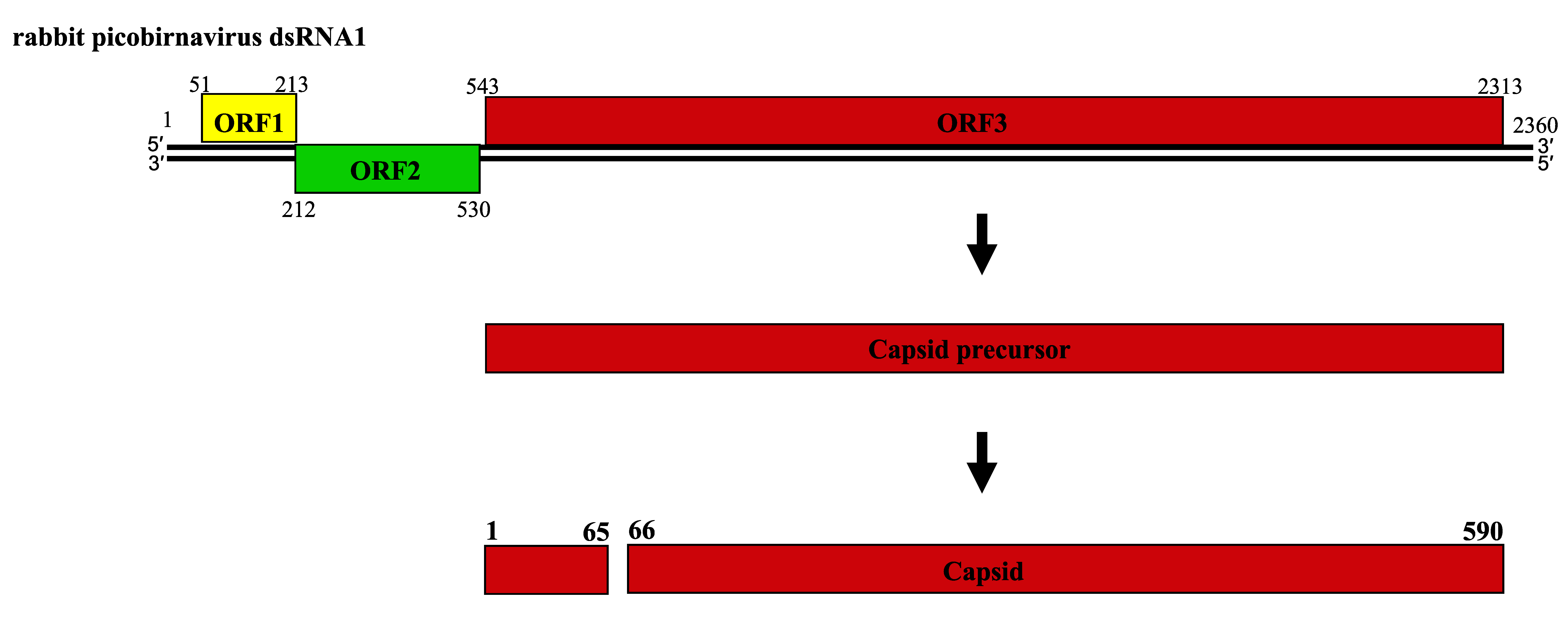 |
| Figure 3. Picobirnaviridae. Schematic representation of the gene arrangement in dsRNA1 of rabbit picobirnavirus and processing of the capsid protein precursor. Numbers indicate the nucleotide positions (in normal type) and the amino acid positions (in bold type). |
The 5′- (GUAAA) and 3′- (ACUGC) terminal nucleotide sequences appear to be conserved in genomic dsRNA2 of picobirnaviruses from various hosts (Wakuda et al., 2005, Ghosh et al., 2009, Malik et al., 2014, Navarro et al., 2017). The consensus bacterial ribosomal binding site sequence (AGGAGG) is present in most of the 5′-untranslated region of picobirnavirus genomes (Krishnamurthy and Wang 2018). Poly-A tract and polyadenylation signal are generally not found in the 3′-untranslated region (Wakuda et al., 2005).
Unsegmented genomes resulting from a fusion of the genomic dsRNA2 at the 3′-end of dsRNA1 (the RdRP gene being located in the 3′-moieties) have been described in vertebrate picobirnaviruses (four isolates from Himalayan marmots [Marmota himalayana], one from horses [Equus caballus], and one from goldsaddle goatfish [Parupeneus cyclostomus]) and invertebrate picobirnaviruses (four isolates from diatom colonies) (Li et al., 2015, Shi et al., 2016, Shi et al., 2018). The RNA-dependent RNA polymerase is active with single strand RNA and double-stranded RNA templates and transcription proceeds in a semi-conservative manner. The RNA-dependent RNA polymerase cannot be incorporated into recombinant capsid in the absence of the viral genome (Collier et al., 2016).
Biology
Natural host range
Picobirnaviruses are widely distributed geographically among humans and mammals in general, and have also been reported in birds and reptiles (Bodewes et al., 2013, Chandra 1997, Fregolente et al., 2009). They have been mainly identified from fecal specimens and in raw sewage samples (Ludert et al., 1991). Picobirnaviruses have also been identified in invertebrates (Shi et al., 2016).
Pathology
The pathogenicity of picobirnaviruses has not been established. Studies conducted with immunocompromised persons suggest that they are opportunistic pathogens that may cause diarrhea (Grohmann et al., 1993, Giordano et al., 1998, van Leeuwen et al., 2010). Picobirnaviruses have been detected in stool samples from children with diarrhea and in immunocompromised patients, and they have also been detected in individuals (and in numerous mammals and birds) lacking signs of gastroenteritis (Malik et al., 2014, Navarro et al., 2017). In individuals with inflammatory bowel diseases or solid-organ transplants, picobirnaviruses are predictive of the occurrence of severe enteric graft-versus-host disease, and correlate with higher fecal levels of severity markers (Legoff et al., 2017). Picobirnaviruses have also been detected in the healthy human and pig respiratory tract (Smits et al., 2011, Smits et al., 2012) and in the plasma of a sick horse (Li et al., 2015). Picobirnaviruses can persistently infect pigs (Martinez et al., 2010).
Transmission
Genetic relationships between picobirnaviruses isolated from different hosts suggest cross-species transmission (Smits et al., 2011, Bányai et al., 2008).
Derivation of names
Picobirna: from Greek pico, “small”; Latin prefix bi, “two”, signifies the bisegmented nature of the viral genome as well as the presence of dsRNA; and RNA abbreviation of ribonucleic acid, indicating the nature of the genome.
Species demarcation criteria
Three virus species are recognized based on their members’ host specificity and the strong sequence divergence of their capsid proteins (originally the only sequence available for a rabbit picornabirnavirus isolate was the dsRNA1 nucleotide sequence). In the future, species demarcation should take in account the apparently wide host specificity amongst viruses infecting mammals, and also integrate the sequence comparison of their complete genomes. Considering the high degree of RdRP and capsid sequences divergence among completely sequenced picobirnaviruses (20 infecting vertebrates and 7 found in invertebrates), each of the picobirnaviruses defined by a completely sequenced genome may eventually be assigned to a separate virus species.
Relationships within the family
Pairwise alignments of RdRP amino acid sequences of completely sequenced picobirnaviruses reveal the existence of three genogroups with high level of sequence divergence in each of these genogroups. The percentage of amino acid identity in pairwise alignments ranged from 29 to 81% in genogroup 1, 29 to 64% for genogroup 2, and 31 to 58% in genogroup 3, whereas the inter-genogroup amino acid identities (between genogroups 1, 2, and 3) ranged from 18 to 25%. Sequence divergence is even more pronounced when amino acid sequences of capsid proteins are compared by pairwise alignments. However, the grouping of the capsid sequences in genetic clusters is difficult to perform since identity scores are low (between 10 to 22%). To date, a single virus has been defined as representative of each genogroup. Phylogenetic analysis of RdRP (Figure 4. Picobirnaviridae) reveals three genetic clusters, two recognized as infecting vertebrates (genogroups 1 and 2) and a third (genogroup 3) with viruses infecting invertebrates. Picobirnaviruses exhibit a high level of genetic diversity, particularly marked within genogroup 1 (Ghosh et al., 2009). Vertebrate picobirnaviruses encode a protein with repeats of the ExxRxNxxxE motif (Da Costa et al., 2011), in contrast to invertebrate picobirnaviruses. Novel picobirna-like RdRP-encoding genome segments using an alternative mitochondrial genetic code constitute a fourth genogroup (Shi et al., 2016, Yinda et al., 2018). Exceptions to this rule are two strains (accession numbers WGML128211 and AWV66966), both of which use a mitochondrial genetic code, despite their sequences clustering with those of picobirnaviruses that use the standard genetic code.
 |
| Figure 4. Picobirnaviridae. A distance tree representing the phylogenetic relationships among RdRPs of picobirnaviruses. The unrooted tree (NJ, complete deletion) was constructed using Mega7 (Kumar et al., 2016). The tree calculation is therefore based only on positions at which an amino acid is present for all sequences in the multiple alignment (ClustalW in Mega 7). Numbers indicate bootstrap values (n=500) for the tree nodes where these were >70%. Sequences are represented by their protein accession numbers. Three RDRP genogroups are indicated. The exemplar isolate of Human picobirnavirus is indicated with a blue spot. This phylogenetic tree and corresponding sequence alignment are available to download from the Resources page. |
Relationships with other taxa
Partitiviruses and picobirnaviruses exhibit similarities in genome organization and RdRP sequences. There is no similarity between picobirnaviruses and birnaviruses, as assessed from differences in their genome organization, capsid structure, and encoded proteins.
Related, unclassified viruses
| Virus name | Accession number |
| otarine picobirnavirus HKG-PF080915 | dsRNA1: JQ776551; dsRNA2: JQ776552 |
| otarine picobirnavirus PF080902 | dsRNA1: KU729754; dsRNA2: KU729755 |
| otarine picobirnavirus PF090307 | dsRNA1: KU729753; dsRNA2: KU729767 |
| roe deer picobirnavirus SLO/D38-14/2014 | dsRNA1: MG190028; dsRNA2: MG190029 |
| marmot picobirnavirus HT4* | dsRNA: KY855431 |
| marmot picobirnavirus HT1* | dsRNA: KY855428 |
| marmot picobirnavirus HT2* | dsRNA: KY855429 |
| marmot picobirnavirus HT3* | dsRNA: KY855430 |
| mouse picobirnavirus 504 | dsRNA1: LC110352; dsRNA2: LC110353 |
| human picobirnavirus CDC23 | dsRNA1: KJ663813; dsRNA2: KJ663814 |
| human picobirnavirus CDC16 | dsRNA1: KJ663815; dsRNA2: KJ663816 |
| human picobirnavirus VS6600008 | dsRNA1: KJ206568; dsRNA2: KJ206569 |
| fox picobirnavirus Fox5 | dsRNA1: KC692367; dsRNA2: KC692366 |
| porcine picobirnavirus 221/04-16/ITA/2004 | dsRNA1: KF861768; dsRNA2: KF861773 |
| equine picobirnavirus Equ1 | dsRNA1: KR902504; dsRNA2: KR902503 |
| equine picobirnavirus Equ2 | dsRNA1: KR902506; dsRNA2: KR902505 |
| equine picobirnavirus Equ3 | dsRNA1: KR902508; dsRNA2: KR902507 |
| equine picobirnavirus Equ4* | dsRNA: KR902502 |
| Beihai goldsaddle goatfish picobirnavirus* | dsRNA: MG600063 |
| Shāhé picobirna-like virus 1* | dsRNA: KX884156 |
| Shāhé picobirna-like virus 2* | dsRNA: KX884154 |
| Běihǎi picobirna-like virus 7** | dsRNA1: KX884063; dsRNA2: KX884062 |
| Běihǎi picobirna-like virus 8 | dsRNA1: KX884065; dsRNA2: KX884064 |
| Běihǎi picobirna-like virus 12* | dsRNA: KX884078 |
| Běihǎi picobirna-like virus 13* | dsRNA: KX884081 |
| diatom colony-associated dsRNA virus 1 | dsRNA1: AP014890; dsRNA2: AP014891 |
Virus names and virus abbreviations are not official ICTV designations.
* unsegmented genome
** Virus representative of the genogroup infecting invertebrates

